![]()
|
| ISSN:1684-193X |
Updated October18, 2004 |
|
| |
|
|
|
|
||||
| Contents: Volume 3, Supplement 1; October, 2004 |
||||
| Yu-Ching Chen, MD; Tzong-Luen Wang, MD, PhD | ||||
|
From the Department of Emergency Medicine (Chen YC, Wang TL), Shin-Kong Wu Ho-Su Memorial Hospital Correspondence to Dr. Tzogn-Luen Wang, Department of Emergency Medicine, Shin-Kong Wu Ho-Su Memorial Hospital, 95 Wen Chang Road, Taipei, Taiwan. E-mail M002183@ms.skh.org.tw
|
||||
|
|
||||
|
|
||||
|
Taiwan's varied landforms contribute to its abundant wild life species. Despite the decreasing number of wild animals caused by the cultivation of forest zone and over hunting on the island, cases of wild animal bite are still reported each year. The focus of care for these cases includes wound care, tetanus immunization, and human-animal communicable diseases prevention and treatment. It is also an important part of wildness medicine. Along with the frequent interactions among Taiwan and other rabies epidemic areas and the rampant smuggling of wild animal in black market, the threat of rabies needs to be critically considered and recognized in Taiwan.
Key words---Wild Animal Injury; Wild Animal Bite; Rabies |
||||
|
|
||||
|
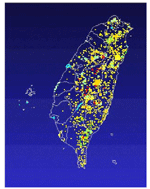
Due to the varied landforms and the subtropical climate, there are 64 species of mammals in Taiwan (Figure
|
|
|||
|
||||
|
||||
The biting wounds caused by wild mammalian can be various. The severity of wounds may span from a simple scratch to more severe punctures, lacerations or avulsions . All can result in significant damage, regardless of the amount of bleeding present. Larger mammalians are able to deliver a bite force of greater than 450 pounds per square inch,4-6 capable of perforating light sheet metal.(Table 2 |
||||
|
||||
The most common complication of bite is infection. Bleeding is another common complication especially when there are vessel injuries. Mammalian teeth may result in a deep laceration, thus creating a route for microorganisms transmission. Osteomyelitis, septic arthritis, tendinitis or tenosynovitis may be seen with tooth penetration into a bone or joint8. Bites of the cranium may yield central nervous system infections such as brain abscess.9 Endocarditis, lymphangitis, meningitis and sepsis with disseminated intravascular coagulation have also been reported. Other infectious complications of mammalian bites include cat-scratch disease (Afipia felis), rat-bite fever (Streptobacillus moniliformis), rabies, tularemia (Francisca tularensis) and tetanus.10 |
||||
Emergency care should be sought immediately if there are serious injuries, the person is suffering from severe blood loss, significant flesh loss, or there are many bites. It is also important to seek emergency care if the person has been bitten by a strange animal. |
||||
1. Open wounds should be irrigated with copious volumes of normal saline as soon as possible. The preferred way is to use high pressure irrigation with normal saline or lactated ringers via a 19 gauge needle and large syringe. |
||||
|
||||
Infections caused by animal bites are often polymicrobial, with an average of three to five bacterial species isolated per wound culture. |
||||
| Prophylactic treatment | ||||
It has been suggested that antibiotic serum concentrations should be therapeutic within 3 hours after the injury. The recommended duration of prophylactic therapy with oral agents is a period of 5-7 days. |
||||
| Empiric antibiotic therapy of infected wounds | ||||
An appropriate empiric antibiotic regimen must direct at the pathogens most likely to cause infection, including both aerobic and anaerobic bacteria. Therapy should target organisms from both the oral cavity of the animal, as well as potential pathogens from the skin flora of the victim. |
||||
|
||||
Several countries, most of which are islands, are rabies free, including the British Isles, New Zealand, Japan, Taiwan, many of the Caribbean islands, Sweden, Norway, and Spain. The fact that these countries remain free of rabies is a tribute to the stringency of their quarantine laws for imported animals. Australia was at one time believed to be rabies free, but bat-transmitted rabies is now endemic there. |
||||
Rabies is caused by a bullet-shaped RNA rhabdovirus that is a member of the Rhabdoviridae family, genus Lyssavirus. Rabies is transmitted by saliva from infected animal bites but may also be transmitted by scratches, secretions that contaminate mucus membranes, aerosolized virus that enters the respiratory tract, and corneal transplants. In recent years, it was noted that rabies could be transmitted from bats to humans by relatively casual contact. The rabies virus has a predilection for nerve tissue and spreads along peripheral nerves and possibly muscle fibers from the contact site to the central nervous system (CNS), causing encephalomyelitis. |
||||
Rabies is a fatal disease once clinical symptoms manifest. Only 6 documented cases survived after onset of clinical rabies. All these patients had received either preexposure prophylaxis or expeditious postexposure prophylaxis after the rabid contact and before the patients had established clinical disease. Rabies presents with 1 of 2 clinical features. Encephalitic (furious) rabies (80%-85% of cases) has the classic presentation with hydrophobia, pharyngeal spasms, and hyperactivity leading to paralysis, coma, and death. The paralytic form is much less common. Incubation periods range from 10 days to 1 year (average, 20-60 days). Prodrome occurs 2 to 10 days after exposure and lasts 1 day to 2 weeks. This stage is characterized by nonspecific flu-like symptoms such as malaise, anorexia, irritability, low-grade fever, headache, nausea, vomiting; paresthesia, pain, or numbness at the bite site. Acute neurologic syndrome occur 2 to 7 days after the prodromes. This syndrome includes dysarthria, dysphagia, excessive salivation, diplopia, vertigo, nystagmus, restlessness, agitation, visual or auditory hallucinations, manic behavior alternating with lethargy, hydrophobia secondary to painful contractions of pharyngeal muscles, polyneuritis; hyperactive deep tendon reflexes with positive Babinski signs and nuchal rigidity often are present. Coma occurs 7 to 10 days after onset of acute neurologic syndrome. This stage is characterized by hydrophobia, prolonged apnea, and generalized flaccid paralysis similar to Guillain-Barre syndrome, seizures, coma, and ultimate respiratory and vascular collapse. Death may follow 2 to 3 days after onset of paralysis but may be delayed by life-support equipments. Recovery is rare. Unfortunately, once the patient is symptomatic, use of antirabies vaccine or rabies immune globulin (RIG) does not improve prognosis, and treatment consists entirely of supportive care. |
||||
Domestic animals that transmit rabies (dogs, cats, cattle) account for only 10% of human exposures, whereas wild animals account for the other 90%, with skunks, foxes, raccoons, and bats being the most prominent. Dogs are the primary reservoir in undeveloped countries. |
||||
Rabies cannot be treated. Therefore, efforts must focuse on prevention. |
||||
Pre-exposure prophylactic immunization is recommended for people who are likely exposed to rabid animals. Veterinarians, animal handlers, and laboratory personnel should consider routine immunization. Also, people traveling to areas where dog rabies is endemic and who will not have easy access to medical care should consider immunization before traveling. A person who was previously immunized and who has had a potential rabies exposure should receive 2 intramuscular doses of vaccine. Give the first dose as soon as possible after exposure and the other 3 days later. |
||||
Passive Vaccination---RIG is a solution of globulins dried from the plasma or serum of selected adult human donors who have been immunized with rabies vaccine and have developed high titers of rabies antibody. |
||||
|
||||
The highest incidence rate of such disease in Taiwan took place in 1956 with the number of one thousand and four identified cases. Ever since the beginning of toxoid vaccination in 1972, the case number has gradually decreased to less than 100 cases. Since 1981, the number of annual reported Tetanus cases has been maintained under 20 cases per year, and the mortality rate has also decreased to single digit number (several cases). |
||||
Approximately, 50% of cases of tetanus in the United States occur after injuries. Infected wounds (both traumatic and surgical) and abscesses, surgical wounds, parenteral drug abuse, major trauma, and animal-related injuries account for 25% of the tetanus-associated injuries. Because immunization is effective in preventing tetanus, the disease is most frequently noted in countries or in ethnic groups whose effective immunization is less likely to be accomplished. |
||||
The tetanus bacillus is a Gram-positive, anaerobic rod that may develop a terminal spore, giving it a drumstick appearance. They can survive in soil for years and may be found in house dust, soil, salt, fresh water, and the feces of many animal species. |
||||
| In addition to neonatal tetanus, tetanus can present in 1 of 3 clinical forms: localized, generalized, or cephalic. We just introduce generalized tetanus here: | ||||
| Generalized tetanus | ||||
It is the most common form of clinical tetanus. It may occur after relatively minor injuries and often follows tetanus-prone wounds. The typical initial findings of trismus due to spasm of the parapharyngeal and masseter muscles are seen in 50% of cases. |
||||
Complications of tetanus include direct toxic effect (laryngeal and phrenic nerves palsy, and cardiomyopathy), and spasm-related sequels (respiratory compromise, rhabdomyolysis, myositis ossificans circumscripta, and vertebral compressed fracture), respiratory compromise (hypoxic cerebral injury), and rhabdomyolysis (acute renal failure), as well as the psychologic impact. |
||||
The classic presenting complaints in tetanus consist of muscle spasms, trismus, stiffness, pain with dysphagia and cranial nerve weakness. These can be seen in other conditions. |
||||
Appropriate treatment based on the clinical diagnosis is warranted even without specific confirmatory laboratory tests. The goals of therapy are eradicating C. tetani, neutralizing its toxin, and providing appropriate supportive care. Specific therapy includes intramuscular administration of tetanus immune globulin (TIG) to neutralize circulating toxin before it binds to neuronal cell membranes. Early administration of antitoxin may prevent spread of the toxin within the CNS. The recommended dosage of TIG ranges from 500 to 3000 U. Additionally, specific therapy includes antimicrobial agents for C. tetani such as penicillin G administered as 200,000 U/kg/d in 4 divided intravenous doses for 10 days. Alternatives for those allergic to penicillin include oral tetracycline (40 mg/kg/d; maximum of 2 g) or intravenous vancomycin (30–40 mg/kg/d).
Local wound care, including surgical debridement, is essential. Foreign bodies should be removed and wounds irrigated well and left open. Patients should be managed in an intensive care setting of a tertiary-care center whenever possible. Facilities and equipments should be available including a quiet darkened room, suction equipment and oxygen, cardiac and respiratory monitors, a ventilator, and tracheostomy equipment. Neuromuscular blockade can be achieved with curariform drugs. The agents used most often are pancuronium and vecuronium. The hypertension that results from sympathetic overactivity may require treatment. Beta-blocking agents are the most useful. Propranolol is administered most commonly. Maintaining adequate nutrition and hydration is of outmost importance. Parenteral nutrition is usually required because of the likely length of the disease and the undesirability of oral or nasogastric feedings. Tracheostomy may be required to prevent laryngospasm. |
||||
The worldwide mortality rate for generalized tetanus ranges from 45% to 55%. Although survivors generally do not experience neurologic sequelae, prolong convalescence with residual muscle rigidity is seen for several months. |
||||
| Active immunization with tetanus toxoid is the most effective mean of protection. The primary series of tetanus toxoid, administered as DTP vaccine at 2, 4, and 6 months and a booster 12 months later, ensures protection in childhood. Additional boosters of tetanus toxoid should be given each decade throughout life.18 | ||||
| Rat bite fever is a systemic illness classically characterized by abrupt onset of fever, rash, and arthralgias, which carries a mortality rate of 13% if left untreated. | ||||
In Asia the causative organism is usually Spirillum minus, a small, spiral gram-negative organism. Children account for more than 50% of rat bite fever cases. In the United States rat bite fever is caused primarily by transmission of Streptobacillus moniliformis, a pleomorphic gram-negative rod . |
||||
| Rat bite fever is a systemic illness characterized by abrupt onset of fever and chills. | ||||
Fevers begin abruptly and usually resolve in 3 to 5 days but can relapse. The rash occurs in roughly 75% of patients and may be maculopapular, petechial, or purpuric. Hemorrhagic pustules or vesicles may also be seen. These usually appear 2 to 4 days after the fever resolves and may last up to 3 weeks. These often involve the extremities, especially the hands and feet. Approximately 20% of rashes due to S. moniliformis will desquamate. Other commonly associated symptoms include severe myalgias, headache, nausea, and vomiting. Within the first week, more than 50% of patients develop a non-suppurative polyarticular or migratory polyarthritis. The joint involvement may affect either large joints or the small joints of the hands and feet. Migratory arthritis may persist for years despite appropriate treatment. |
||||
The incubation period averages 14 to 18 days, with a range of 1 to 36 days. If there is a bite, infection is usually heralded by an indurated lesion at the site as symptoms become obvious. The lesions may ulcerate and there may be regional lymphadenopathy. The fevers have regular relapses separated by afebrile periods lasting 3 to 7 days. Approximately 50% of patients develop a violaceous red-brown rash that usually consists of large macules with occasional erythematous plaques or urticarial-type lesions. The joint manifestations are rare. The mortality with S minus infection is slightly lower than that seen with S moniliformis and approaches 6.5%.
If infection with either organism goes unrecognized, there can be serious sequelae, including arthritis, endocarditis, myocarditis, pericarditis, pericardial effusion, hepatitis, nephritis, and meningitis. |
||||
| Blood culture isolation is the gold standard. | ||||
Penicillin is the treatment of choice despite rare reports of penicillin-resistant strains. Current recommended treatment is 5 to 7 days of intravenous penicillin at doses of 20,000 to 50,000 units/kg/day followed by 7 days of oral penicillin.19 |
||||
As we protect wild-animal species and acknowledge their right to share territory, interactions—and possibly attacks—are likely to increase. Awareness, education, knowledge and prevention, rather than the elimination of animal populations, may be the best way to control wild-animal attacks on humans in the future. |
||||
|
|
||||
| 1. |
????????????????????????????????,1994,?? |
|||
| 2. | ???????????????????????????????????????????????(?????????),1992,??? | |||
| 3. | ??????????40????????---??????? 2002/07/12 21:15 | |||
| 4. | Lewis KT, Stiles M. Management of cat and dog bites. Am Fam Physician 1995;52:479-85 | |||
| 5. | Lewis KT, Stiles M. Management of cat and dog bites. Am Fam Physician 1995;52:479-85 | |||
| 6. | Garcia VF. Animal bites and pasturella infections. Pediatr Rev 1997;18:127-30 | |||
| 7. | Goldstein EJC, Citron DM, Finegold SM. Dog bite wounds and infection: a prospective clinical study. Ann Emerg Med 1980; 9:508-512 | |||
| 8. | Brogan TV, Bratton SL, Dowd MD, Hegenbarth MA. Severe dog bites in children. Pediatrics 1995;96:947-950 | |||
| 9. | Agahbabian RV, Conte JE. Mammalian bite wounds. Ann Emerg Med 1980;9:79-82. | |||
| 10. | Goldstein EJC. Bite wounds and infection. Clin Infect Dis 1992;14:633-640 | |||
| 11. | Smith PF. Treating mammalian biting wound, Journal of clinical Pharmacy and Therapeutics. 2000;25:85-99 | |||
| 12. | Hankins DG. Rosekrans JA. Overview, prevention, and treatment of rabies Majo Clin Proceedings 2004;79: 671-76 | |||
| 13. | Weir E. Putting the bite on rabies. CMAJ 2002;167:781 | |||
| 14. | Jackson AC. Update on rabies. Curr Opin Neurol 2002;15:327-31 | |||
| 15.. | Kaeser HE, Sauer A. Tetanus toxin: a neuromuscular blocking agent. Nature 1969;223:842 | |||
| 16. | Einstein L. Tetanus. N Engl J Med. 1973;289:293–96 | |||
| 17. | Itzhak B. Tetanus in children. Ped Emerg Care 2004;20,48-51 | |||
| 18. | Freels LK, Elliott SP. Rat bite fever: three case reports and a literature review. Clin Ped 2004;43:291-5 | |||
| 19.. | Norwood.S. Mechanisms and Patterns of Injuries Related to Large Animals. J Trauma 2000;48:740-744 | |||